For imaging of newly diagnosed or recurrent prostate cancer
MECHANISM OF ACTION
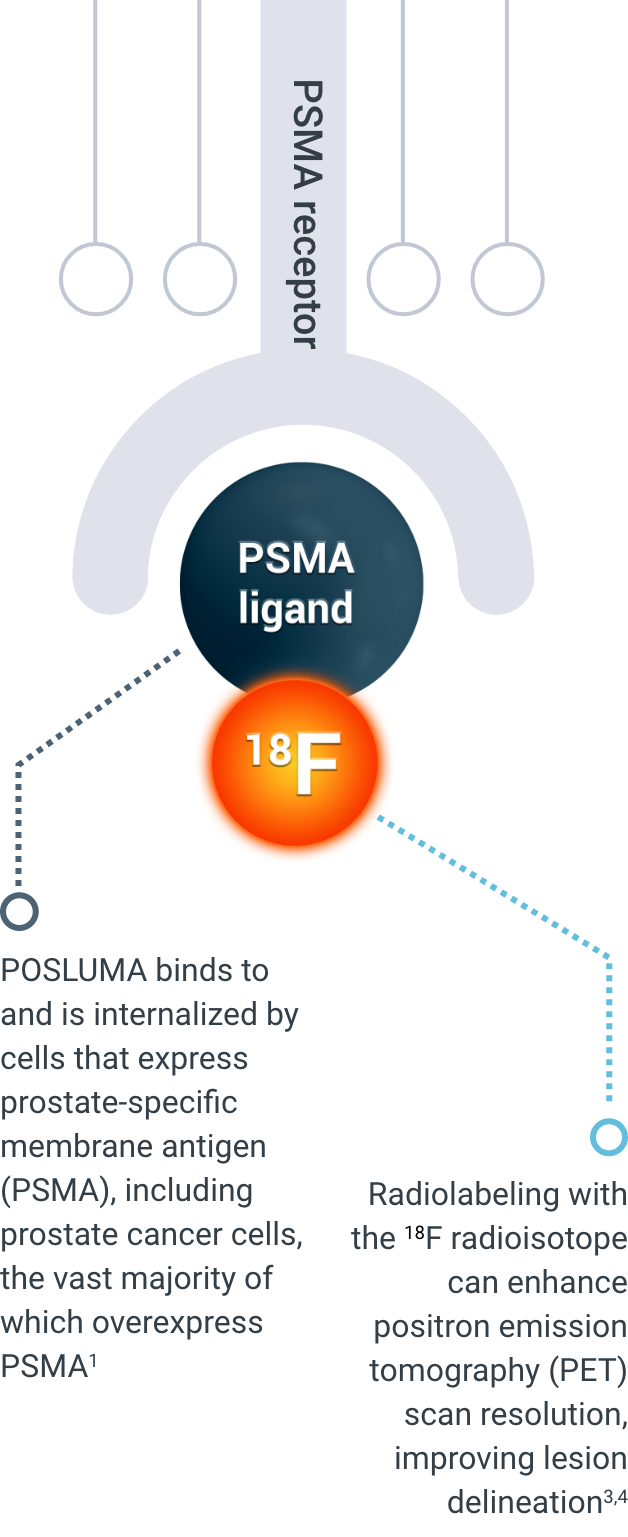
How POSLUMA works
Explore POSLUMA’s precise PSMA-targeting mechanism of action
IS SOMETHING OBSCURING YOUR PSMA SCANS?
Activity in the urinary bladder can impact the assessment of tumors and lymph nodes local to the prostate region (the most common site of recurrence), sometimes producing halo artifacts that deteriorate image quality.7-9
LOW URINARY BLADDER ACTIVITY
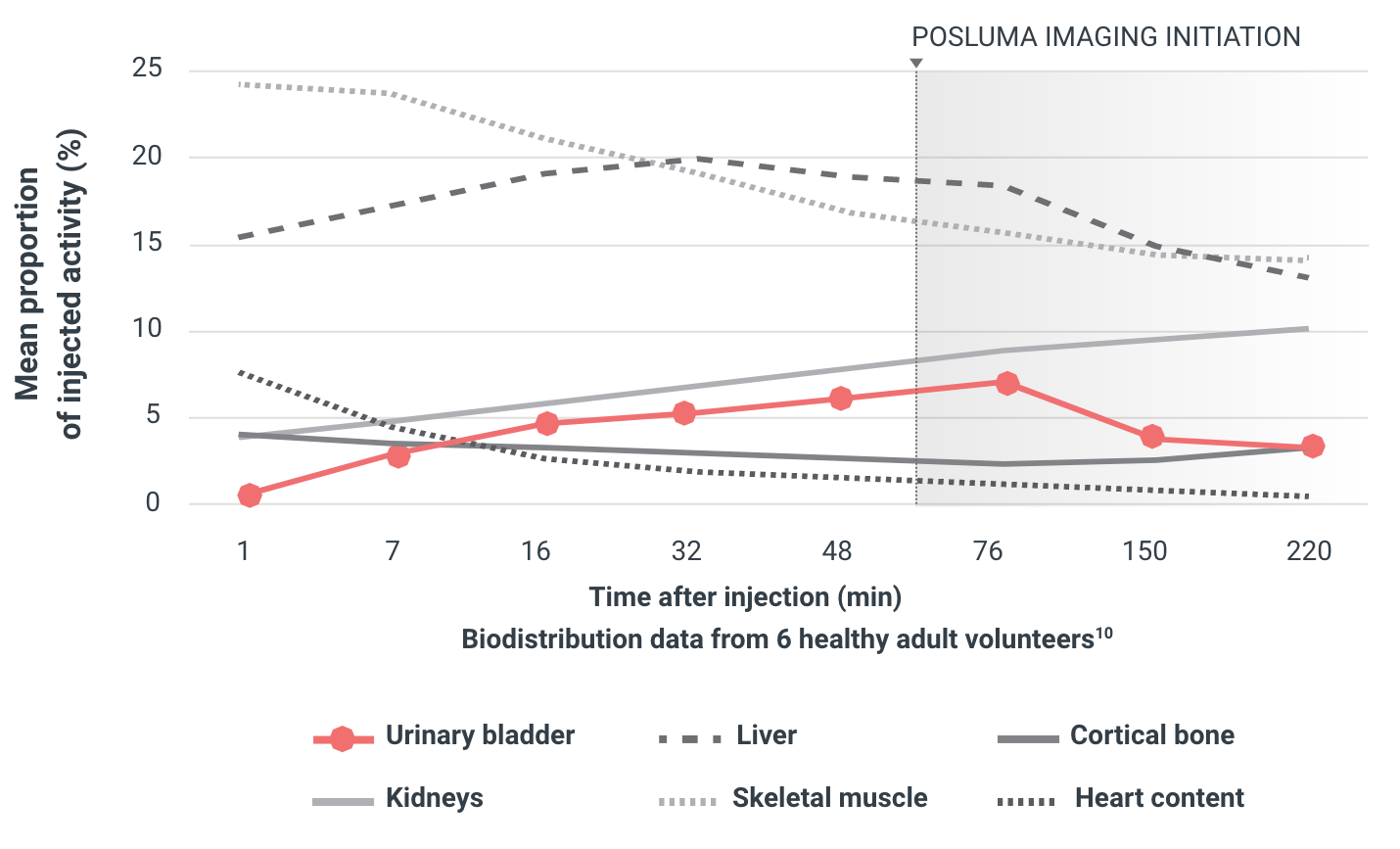
In preclinical and Phase 1 studies, low radioactivity in the urinary bladder improved tumor-to-background ratio and may enhance confidence in image interpretation in the prostate bed and surrounding areas.7,10
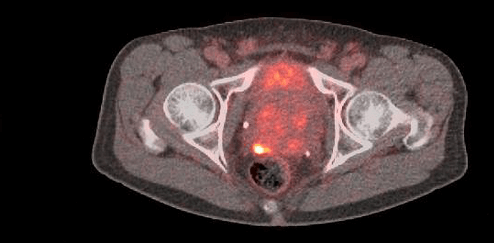
PET images of a healthy volunteer after administration of POSLUMA.
The first scan session was at 1 to 90 minutes after injection, the second was at 150 to 178 minutes, and the third was at 220 to 248 minutes. Subject was permitted to leave PET scanner and void urine between sessions.
References: 1. POSLUMA. Package insert. Blue Earth Diagnostics Ltd; 2023. 2. Donin NM, Reiter RE. Why targeting PSMA is a game changer in the management of prostate cancer. J Nucl Med. 2018;59(2):177-182. doi:10.2967/jnumed.117.191874 3. Werner RA, Derlin T, Lapa C, et al. 18F-labeled, PSMA-targeted radiotracers: leveraging the advantages of radiofluorination for prostate cancer molecular imaging. Theranostics. 2020;10(1):1-16. doi:10.7150/thno.37894 4. Rowe SP, Drzezga A, Neumaier B, et al. Prostate-specific membrane antigen–targeted radiohalogenated PET and therapeutic agents for prostate cancer. J Nucl Med. 2016;57(suppl 3):90S-96S. doi:10.2967/jnumed.115.170175 5. Jani AB, Ravizzini GC, Gartrell BA, et al. Diagnostic performance and safety of 18F-rhPSMA-7.3 PET in men with suspected prostate cancer recurrence: results from a phase 3, prospective, multicenter study (SPOTLIGHT). J Urol. Published online April 26, 2023. doi:10.1097/JU.0000000000003493 6. Wurzer A, Di Carlo D, Schmidt A, et al. Radiohybrid ligands: a novel tracer concept exemplified by 18F- or 68Ga-labeled rhPSMA inhibitors. J Nucl Med. 2020;61(5):735-742. doi:10.2967/jnumed.119.234922 7. Knorr K, Oh S, Krönke M, et al. Preclinical biodistribution and dosimetry and human biodistribution comparing 18F rhPSMA 7 and single isomer 18F rhPSMA 7.3. EJNMMI Res. 2022;12(8):8. doi:10.1186/s13550-021-00872-w 8. Expert Panel on Urologic Imaging; Froemming AT, Verma S, Eberhardt SC, et al. ACR Appropriateness Criteria® Post-treatment Follow-up Prostate Cancer. J Am Coll Radiol. 2018;15(5S):S132-S149. 9. Oh SW, Wurzer A, Teoh EJ, et al. Quantitative and qualitative analyses of biodistribution and PET image quality of a novel radiohybrid PSMA, 18F-rhPSMA-7, in patients with prostate cancer. J Nucl Med. 2020;61(5):702-709. doi:10.2967/jnumed.119.234609 10. Tolvanen T, Kalliokoski K, Malaspina S, et al. Safety, biodistribution, and radiation dosimetry of 18F-rhPSMA-7.3 in healthy adult volunteers. J Nucl Med. 2021;62(5):679-684. doi:10.2967/jnumed.120.252114