LIGHTHOUSE was an open-label, prospective, Phase 3, multicenter, single-dose imaging study uniquely assessing the safety and efficacy of POSLUMA PET imaging in 356 men with newly diagnosed unfavorable intermediate-risk, high-risk, or very high-risk prostate cancer.1-3 Please see additional study design below.
COPRIMARY ENDPOINTS
The coprimary endpoints were sensitivity and specificity of POSLUMA PET imaging for detecting N1 disease in concordance with histopathology of the pelvic lymph node dissection.2,3
Out of 296 patients with sufficient histopathology data for evaluation of the pelvic lymph nodes, across 3 readers1
SPECIFICITY
Patient-level
93% to 97%
Across 3 readers
SENSITIVITY
Patient-level
23% to 30%
Across 3 readers
Majority read sensitivity and specificity compared to histopathology (N=296)2,3
SPECIFICITY
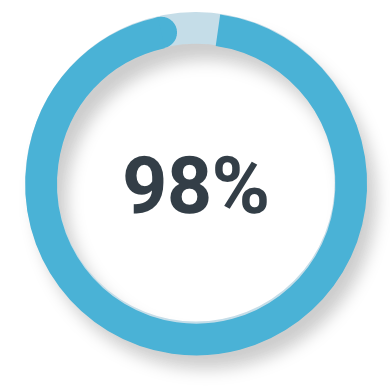
Unfavorable intermediate-risk prostate cancer
Majority read
(78/80; 95% CI: 91%, 100%)
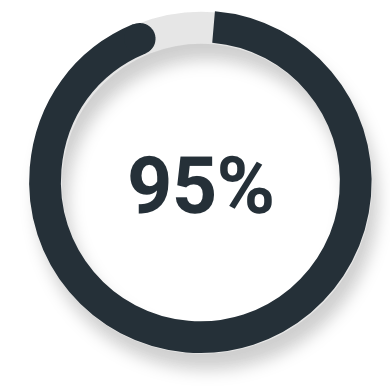
High-risk or very high-risk prostate cancer
Majority read
(139/146; 95% CI: 90%, 98%)
SENSITIVITY
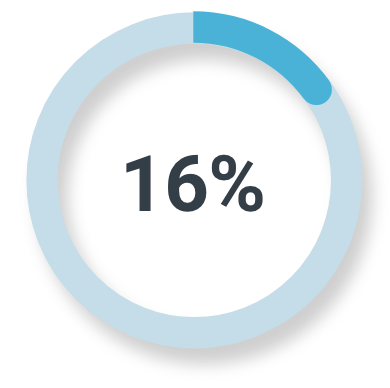
Unfavorable intermediate-risk prostate cancer
Majority read
(3/19; 95% CI: 3%, 40%)
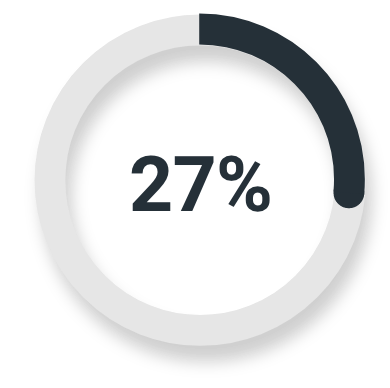
High-risk or very high-risk prostate cancer
Majority read
(14/51; 95% CI: 16%, 42%)
Histopathology as the standard of truth
PET may detect lesions as small as 4 to 5 mm, but histopathology can detect micrometastases.4,5
HOW DOES DISTANT METASTATIC DISEASE IMPACT YOUR INITIAL TREATMENT PLAN?
In recent years, the rate of identified metastatic prostate cancer cases has risen from 4% to 8% with fewer than one-third of men surviving 5 years after a diagnosis of distant metastatic disease. However, survival is on the rise, which may reflect changes in clinical management and treatment options.6‑8 POSLUMA’s proven metastatic lesion detection can provide crucial information to guide a patient’s treatment plan.1,3
N1 AND M1 DETECTION
POSLUMA detected N1 and M1 lesions, even in patients with unfavorable intermediate-risk prostate cancer.3
*Percent PET positivity.
MAJORITY READ
N1 disease
13%
of patients
had ≥1 pelvic lymph node metastasis (N1) detected3*
(47/352; 95% CI: 10%, 17%)
M1 disease
17%
of patients
of patients had ≥1 extrapelvic site metastasis (M1) detected3*
(61/352; 95% CI: 14%, 22%)
VERIFIED DETECTION RATE
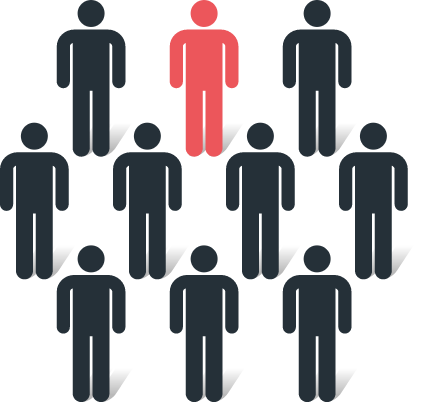
1 in 10 patients
(34/352) had at least 1 verified distant metastatic lesion revealed by POSLUMA1*
56% (34/61; 95% CI: 42% to 68%) of patients with ≥1 distant metastatic lesion detected were confirmed by majority read and a reference standard.1*
*Histopathology or confirmatory imaging was used as the standard of truth for M1 disease evaluation.
References: 1. POSLUMA. Package insert. Blue Earth Diagnostics Ltd; 2023. 2. Chapin B; LIGHTHOUSE Study Group. Diagnostic performance and safety of 18F-rhPSMA-7.3 PET in patients with newly diagnosed prostate cancer: results from a phase 3, prospective, multicenter study (LIGHTHOUSE). Poster presented at: 23rd Annual Meeting of the Society of Urologic Oncology. December 1, 2022. San Diego, CA. 3. Data on file. LIGHTHOUSE clinical study report. Blue Earth Diagnostics, Ltd. Oxford, UK. 4. Magi-Galluzzi C. Prostate cancer: diagnostic criteria and role of immunohistochemistry. Mod Pathol. 2018;31(S1):S12-S21. doi:10.1038/modpathol.2017.139 5. Adler S, Seidel J, Choyke P, et al. Minimum lesion detectability as a measure of PET system performance. EJNMMI Phys. 2017;4(1):13. doi:10.1186/s40658-017-0179-2 6. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708 7. American Cancer Society. Cancer Facts & Figures 2022. Atlanta, GA: American Cancer Society; 2022. Accessed December 16, 2022. https://www.cancer.org/research/cancer-facts-statistics/all-cancerfacts-figures/cancer-facts-figures-2022.html 8. Siegel DA, O’Neil ME, Richards TB, Dowling NF, Weir HK. Prostate cancer incidence and survival, by stage and race/ethnicity - United States, 2001-2017. MMWR Morb Mortal Wkly Rep. 2020;69(41):1473-1480. doi:10.15585/mmwr.mm6941a1